OVERVIEW OF COMPANY
Founded in 2017, Connext is a clinical-stage biotech company developing biological therapeutics (medicinal recombinant protein products) to improve quality of life. Based on recombinant TLR5 agonist technology, Connext is developing a therapeutic agent (first-in-class) to prevent side effects occurring during radiation and chemotherapy. Connext is also developing the world's first recombinant collagenase treatment. Connext is expanding its pipeline in the field of immuno-oncology and regenerative medicine and striving to develop and commercialize them.
HISTORY
MISSION AND VALUE
Connext is committed to improving quality of life by rapidly delivering innovative medicines and technologies to patients in the field of regenerative medicine through continual connect and innovation.
ORGANIZATION
Board of
Directors
CEO
SAB
Technology (CTO)
- R&D
- Process Development/scale-up
- IP Management
Quality (CQO)
- Method Development
- Quality Management
- GMP Mfg Management
Development (CDO)
- Pre-clinical/clinical Development
- Regulatory Affairs
Communication (CCO)
- PR
- Communication Channel
- Product Marketing
- Business Development
Operation (COO)
- IR/Finance
- Accounting
- Human Resource
INVESTORS
PIPELINE
| Nonclinical | Phase 1 | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|---|
| Recombinant TLR5 agonist | CNT101 | Acute graft-versus-host disease (aGVHD) | ||||
| Acute radiation syndrome | ||||||
| Oral mucositis | ||||||
| CNT102 | Cancer immunotherapy | |||||
| Recombinant collagenase | CNT201 | Dupuytren’s contracture | ||||
| Peyronie’s disease | ||||||
| Cellulite | ||||||
| CNT202 | Wound debridement | |||||
TLR, TLR5 agonist
The TLR family plays a fundamental role in pathogen recognition and activation of innate immunity. TLRs are highly conserved from Drosophila to humans and share structural and functional similarities. They recognize pathogen-associated molecular patterns (PAMPs) that are expressed on infectious agents, and mediate the production of cytokines necessary for the development of effective immunity. The various TLRs exhibit different patterns of expression.
Toll-like receptor 5, also known as TLR5, is a protein which in humans is encoded by the TLR5 gene. TLR5 is known to recognize bacterial flagellin from invading mobile bacteria.
Collagenase
The most abundant protein in mammals, Collagen is a fibrous component in connective tissue. Collagenase is an enzyme derived from microorganisms that breaks down collagen. (Two types of Collagenase: class I and class II)
MILESTONES
TLR5 agonist
Collagenase
-
2022
-
Dupuytren’s contracture
Phase 1/2 Pre-IND
completed (FDA) -
2023
-
Acute graft-versus-host
disease Phase 1 IND
approved (MFDS) -
Dupuytren’s contracture
Phase1/2 IND
approved (FDA) -
2024
-
Initiating Dupuytren’s
contracture Phase 1/2
clinical trials (Australia) -
Initiating acute
graft-versus-host
disease clinical
trials (MFDS) -
2025
-
Peyronie’s Disease
pre-IND planned -
Dupuytren’s
contracture
phase 1/2 clinical
trials completed
BUSINESS MODEL
Promotion of commercialization through product sales rather than simple technology transfer
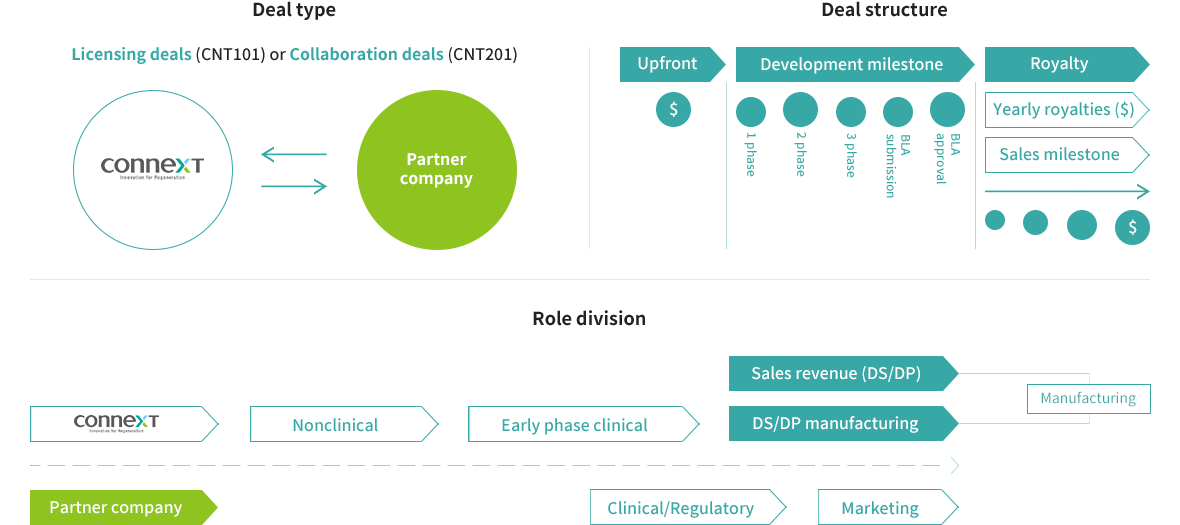

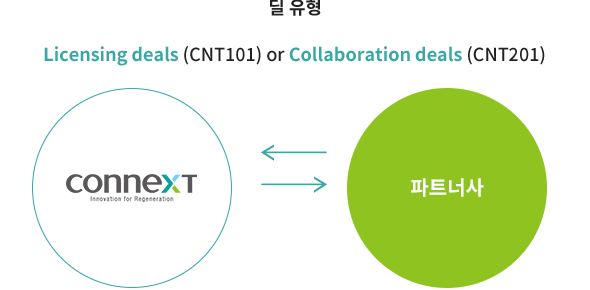
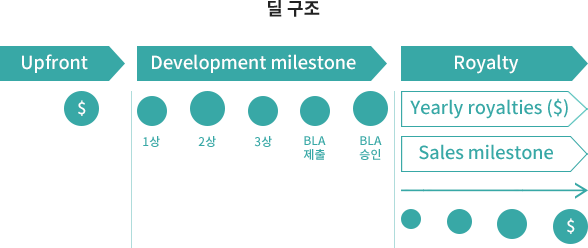
LOCATION
Suite 1701, 322 Teheran-ro Gangnam-gu, Seoul, Republic of Korea
Suite C-03, 6th floor, Seoul National University Bundang Hospital Healthcare Innovation Park 172, Dolma-ro,
Bundang-gu, Seongnam-si, Gyeonggi-do, Republic of KOREA



